|
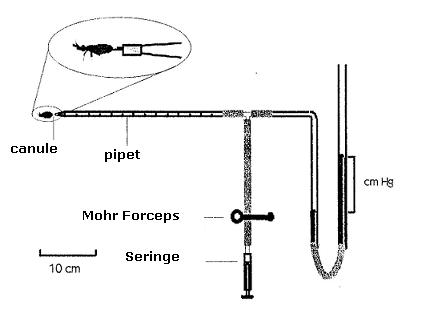
Procedure
1- Preliminary Manipulations:
a. Remove the syringe.
b. Remove graduated
pipette and remove the cannula.
c. Fill the pipette with
some Ringer solution until slightly surpassing the graduated zone
and reconnect.
d. Reconnect the cannula
in the pipette and fill it up by pressing the rubber hose until the
meniscus reaches
the graduated zone,
and this way filling up the cannula with Ringer solution.
e. Remove the cannula.
f. Analyze and discuss
the results
Recommendations
- During the manipulation of the
graduated pipette and the cannula, work with your elbows well
supported on the table, also maintaining the fingers in both hands
close together.
- When the pipette or cannula are
filled, maintain them horizontally to avoid any loss of the
solution.
- If necessary, it is possible to
make the plastic cannula bigger with a needle to introduce it in the
graduated pipette.
- If the assassin bug defecates
during the insertion of the cannula, dry the affected zone with
absorbing paper until the anus is clearly visible.
- Handle the piston with care and
avoid making sudden movements.
Bibliography
Bennet-Clark, HC (1962). Active
control of the mechanical properties of insect endocuticle. J.
Insect Physiol.8: 627-633
Nunez, JA (1963). Central nervous
control of the mechanical properties of the cuticle in Rhodnius
prolixus. Nature, 199: 621-2
Maddrell, SHP (1966). Nervous
control of the mechanical properties of the abdominal wall at the
feeding in Rhodnius. J. Exp. Biol. 44: 59-68
Reynolds, SE (1985). Hormonal
control of cuticle mechanical properties in Kerkut & Gilbert (eds.)
Nervous System: Sensory (Comprehensive Insect Physiology,
Biochemistry and Pharmacology, vol VI), 335-351
Ianowski, J.P.; Manrique, G.;
Nunez, J.A> & Lazzari, C.R. Feeding is not necessary for triggering
plasticization on the abdominal cuticle in haematophagous bugs (en
prensa en J. Insect Physiolo.) (1998).
2- First Experimental Series:
a. Identify and select a
group of abdominal sections in the larvae of the 5th state (make a
graph of their
distribution).
Measure the distance between the bases of those sections using a
graduated scope.
b. Feed the assassin bugs
with the cow's blood for about 1 minute, assuming that it starts
feeding when it
does not separate its
mouth from the latex membrane.
c. Immobilize the
assassin bug face down on a bough of magnets. With the aid of a
magnifying glass, insert
the cannula in the
anus of the assassin bug (approx. 2mm).
d. Seal with paraffin.
This is done by taking the paraffin scales with the micro cauterizer
and placing them
over the metal cannula
on the zone adjacent to the anus.
e. Place the assassin bug
(already connected to the cannula) in the pipette (facing down).
-Measurements:
a. Prepare the table of
values (volume vs. time).
b. Apply pressure of 10
cm of Hg with the syringe and place the Mohr forceps to ensure that
it remains
constant.
c. Measure the flow,
volume/minute (same person always measuring) of the flow of Ringer
solution inside
the assassin bugs.
d. Measure again the
distance between the bases of the identified abdominal sections.
3- Second Experimental Series:
Repeat the procedure in steps a, c,
d, and e of the first experimental series (using this time bugs not
fed) and all
the steps in the measurements.
4- Finalizing and analyzing the
data
a. Remove the assassin
bug, the remaining paraffin and the remaining solution from the
pipette (be cautious
with the escape of any
remaining solution).
b. Remove the syringe and
proceed with the cleaning of the cannula (with a copper wire) and
the cleaning
of the pipette (as
long as it is necessary).
c. Make the volume vs.
time graphs for both experimental series.
>>Continue <<Back
<<Back to IPO |
